
Retinoids have been used both as pharmaceuticals and cosmetics for many years. There is a significant amount of data for all forms of Vitamin A, and, as a topical preparation, retinoids are widely used for dermatological disorders, such as plaque psoriasis, acne and oily skins and photoageing. This article will review the various forms of Vitamin A, how they work and the differences in actions, as well as their relevant uses and clinical evidence.
History of retinoids
The use of retinoids for topical treatment of acne and photoageing has increased in recent years, both in pharmaceutical and cosmetic arenas; however, as with other key topical ingredients for photoageing, Vitamin A has actually been in use for centuries—it has been reported that liver was used to treat night blindness in ancient Egypt (Mukherjee et al, 2006). More recent medical literature reports the use of retinoids for both topical and systemic treatment of cutaneous disorders.
For example, Karrer described the chemical structure of retinol derived from shark liver oil in 1931, for which he received the Nobel Prize (Baumann, 2009). Stüttgen reported efficacy with retinoids in treating hyperkeratotic conditions—such as lamellar ichthyosis and actinic keratosis—in 1962, and went on to report the use of topical tretinoin for acne vulgaris in 1969. An international symposium held in Flims, Switzerland in 1975 demonstrated keen awareness of the therapeutic potential of tretinoin and the rapid growth of basic knowledge of the pharmacology of retinoids, and, as a result, drove further research into new applications for topical retinoids, such as the treatment of flat warts, lichen planus, Darier's disease, as well as the prevention and treatment of photoageing (Stüttgen, 1986).
In 1993, Biro et al wrote about the use of retinoids in three key areas where they are believed to have major impact: acne vulgaris, photoageing and cutaneous neoplasia (Biro and Shalita, 1993). The use of retinoids for psoriasis and other hyperkeratotic and parakeratotic skin disorders, keratotic genodermatoses, severe acne and acne-related dermatoses, and also for therapy and/or chemoprevention of skin cancer and other neoplasia, is discussed by Orfanos et al in a 1997 paper titled ‘Current use and future potential role of retinoids in dermatology’ (Orfanos and Zouboulis, 1997). Chronologic ageing and photoageing are cosmetically relevant, responsible for psychological changes and are implicated in the development of pre-cancerous and cancerous lesions, and are also responsible for causing functional fragility in the skin. Vitamin A has been associated with dermatoporosis since the word dermatoporosis was coined in 2007 by Kaya and Saurat to describe this functional fragility (Kaya and Saurat, 2007).
The volume of literature now available for retinoids is immense, much of it centered around specific formulations as manufacturers and cosmetic scientist search for a balance of efficacy and safety in treating a range of cutaneous disorders and treating and preventing ageing processes. Retinoids can be categorised by generation, as demonstrated in Figure 1.
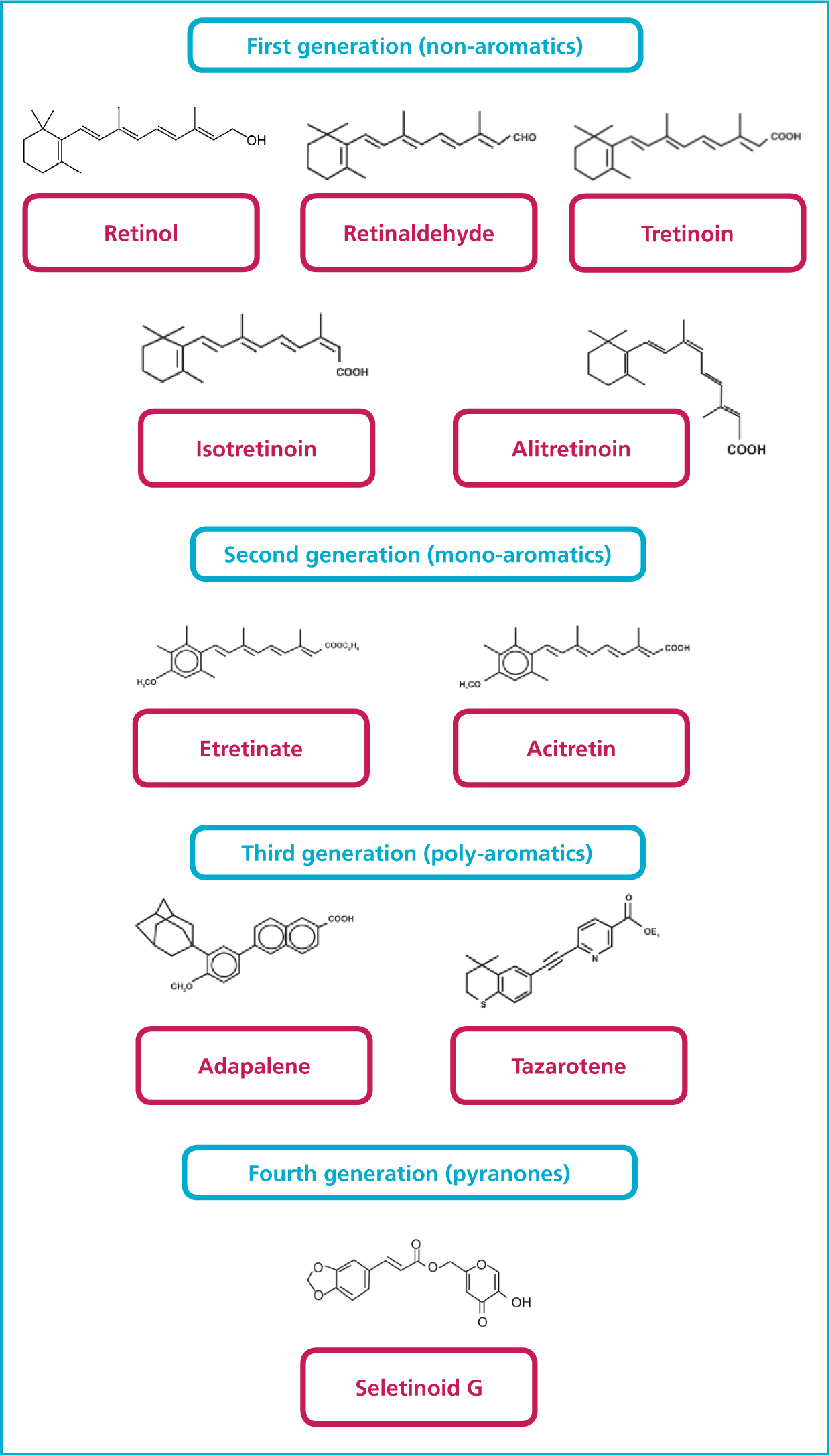
How do retinoids work?
Retinoids cover a huge spectrum of products, from cosmetics to prescription only medicines. Sorg et al described this simply in Chapter 8 of Cosmetic Dermatology: Products and Procedures as a ranking order of retinoid-like activity as a result of topical application as follows: retinoic acid > retinaldehyde > retinol > retinyl esters (Draelos, 2016). It is important to note that Vitamin A cannot be synthesised by the body, but needs to be supplied to the body in a ‘ready to use’ form (Mukherjee et al, 2006). The scope of this article is to look at topical forms of Vitamin A, but the author notes that retinol and retinyl palmitate, the dietary forms of Vitamin A, are widely available. Retinyl palmitate is in many foods, such as beef and chicken liver, eggs, fish liver oils, whole milk, yogurt, butter, and cheeses. Beta-carotene and carotenoids can be converted in the body to make vitamin A, and are found in fat-soluble nutrients in dark-green leafy vegetables and deep yellow/orange vegetables, such as sweet potatoes, carrots, pumpkin and other winter squashes, cantaloupe, apricots, peaches, and mangoes (University of Rochester Medical Center, 2019).
Commercially available synthesised retinol has been available since the 1940s and, by the turn of the millennium, there were over 2500 retinoid products available (Kligman, 1998). There are also combination products available with other prescription strength ingredients such as hydroquinone, for example in Tri-Luma, and many cosmetic preparations have retinoids combined with synergistic ingredients.
Retinoic acid (RA) can be irritating to the skin, limiting its use in some patients. Retinol and retinaldehyde are relevant to the aesthetic and skin care markets as they are gentler yet still effective alternatives to RA. However retinyl palmitate and retinyl acetate, both cosmetic retinoids, are retinoid esters and are not considered effective against photoageing (Levin and Momin, 2010), and therefore are not discussed in this article.
Vitamin A is required for many biological processes and is present in the body as retinyl esters and betacarotene; the retinyl esters are stored in the liver, having been converted to retinol before absorption in the intestine. For circulation, retinol is bound to proteins known as plasma–retinol binding proteins. The main areas where retinoids are essential are reproduction, embryogenesis, growth, vision, the inflammatory process and cell differentiation, proliferation and apoptosis (Mukherjee et al, 2006). Retinol and retinyl esters make up 99% of cutaneous retinoids (Draelos, 2016).
There are complex mechanisms of action affecting various cellular processes, such as cellular differentiation and immune modulation. Many of these tissue effects are related to interaction with specific cellular and nucleic acid receptors. The work done since the discovery of retinoid binding proteins in the 1970s led to the discovery, in 1987, that tretinoin is in fact a hormone (Giguere et al, 1987). It is now understood that various binding proteins mediate the effect of retinoic acid: these are cellular retinoic acid binding proteins I and II (CRABP I and II), cellular retinol binding proteins (CRBP) and two categories of nuclear receptors—retinoic acid receptors (RARs) and retinoid X receptors (RXRs) (Baumann, 2009). The interactions of retinoid receptors with other retinoid receptors and with members of the nuclear hormone family are complex, and there is much ongoing study and research underway.
Retinoid actions
In order to understand the effects of retinoids on various dermal symptoms of photoageing, we must consider all the effects of retinoids.
Fine lines and wrinkles
Retinoids are widely used to address fine lines and wrinkles, and have been shown to increase the water content of the dermis by stimulating glycosaminoglycan (GAG) synthesis and by stimulating both transforming growth factor (TGF=beta) procollagen, leading to an increase in dermal collagen, particularly Type I. In increase in collagen anchoring fibils, Collagen Type VII, has also been demonstrated (Kang et al, 1997; Sorg et al, 2006; Bermann, 2007; Ligade et al, 2009; Baumann, 2009). Further actions of retinoic acid are the inhibition of matrix metalloproteinases, a collagen degrading protein produced as a response to Ultraviolet B (UVB) exposure, leading to prolonged tissue residency of dermal collagen, and the prevention of oxidative stress (Tsuchiya et al, 1992; Tesoriere et al, 1997; Singh and Lippman, 1998; Sorg et al, 2001; Sorg et al, 2005; Bermann, 2007).
Skin roughness
Retinoids promote epidermal cell turnover by gene modulation of expression of genes involved in cellular differentiation and proliferation (Kang et al, 1997; Bermann, 2007).
Hyperpigmentation
Retinoic acid enhances cell turnover, which in turn decreases contact time between keratinocytes and melanocytes, thereby promoting loss of pigment through epidermopoiesis, and reducing clumping of melanin in basal cells (Sorg et al, 2006; Baumann, 2009). Retinoic acid has been shown to have a direct tyrosinase inhibitory effect in some studies, but not others (Hoal et al, 1982; Edward and Gold, 1988) and has led to the conclusion that effects in general on hyperpigmentation are related to epidermal turnover rates, and not direct effects on melanin content or melanocyte growth.
Photodamage
Retinoids absorb UV light, which allows topical retinoids to act as UV filters. A study of topical retinyl palmitate demonstrated equivalent effects in prevention of UVB-induced erythema and DNA photodamage. The study used mouse skin in vitro, and results demonstrated that epidermal retinyl esters have a biologically relevant filter activity (Antille et al, 2003).
Antibacterial properties
Retinaldeheyde has been shown to have direct biological effect on biomolecules on the skin surface as well as on bacterial flora, and has been successfully used against cutaneous grampositive bacteria, and was seen to be more potent than retinol and retinoic acid (Pechère et al, 1999; Pechère et al, 2002).
Oxidation
Topical retinoids have been shown to have antioxidant, free-radical scavenging properties in vitro (Sorg et al, 2001), and Draelos suggests their use alongside other topical antioxidants to ensure full antixodiant protection (Draelos, 2016).
Types of retinoids
Tretinoin
Tretinoin has been used as a topical treatment in dermatology for many years, and there is a vast bank of clinical data behind its use in both dermatology and for the treatment of photoageing. Kligman et al used an animal model to demonstrate collagen deposition in the papillary dermis with a direct correlation to wrinkle effacement (Kligman et al, 1984). Later ex-vivo research demonstrated a complete blockade of interstitial colleganase and gelatinase synthesis preventing collagen degradation, as well as reducing UV-induced activation of two nuclear transcription factors (Fisher et al, 1996). Kligman went on to demonstrate, in 1986, clinical improvement of photoaged skin in a vehicle-controlled open study using 0.5% tretinoin applied topically to photoaged forearm and facial skin. Histological examination of the treated areas showed increased deposition of reticulin fibres and neocollagenesis of types I and III, as well as angiogenesis in the papillary dermis (Kligman et al, 1986).
Isotretinoin
Isotretinoin has been shown to reduce the visible symptoms of photoageing, such as fine lines and wrinkles and discrete pigmentation, sallowness and texture without causing significant irritation (Armstrong et al, 1992; Sendagorta et al, 1992). Histological studies have demonstrated an increase in viable epidermal thickness (VET), but failed to demonstrate significant changes in parameters such as dermal elastosis, dermal thickness, epidermal melanin content, number of fibroblasts, and melanocyte dysplasia or keratinocyte atypia. Up to 10% of patients experienced severe irritation, particularly on facial skin; most of the other patients experienced only mild irritation (Maddin et al, 2000).
In 2005, Griffiths et al presented a 6-month multicentre, double blind, randomised, parallel-group, vehicle controlled study of 346 subjects with photoaged skin using a 0.5% isotretinoin combined with sunscreen. Of the 346 subjects, 172 received active ingredients, the remaining 174 receiving vehicle only. The main outcome measure was profilometry measurements of replicas of the periorbital region, taken at 0 and 6 months. Subsidiary outcome measures were clinical scoring of wrinkles/fine lines at 0, 1, 3, 6 and 9 months and histopathology at 0 and 6 months (Griffiths et al, 2005). At all visits, clinical scoring of wrinkles/fine lines showed a statistically significant difference between the groups in favour of Isotretinoin (Griffiths et al, 2005).
Retinol
Retinol needs to be converted to all-trans-retinoic acid via retinaldehyde within keratinocytes to exert an effect on the epidermis, and although it is 20 times less potent than tretinoin (Kurlandsky et al, 1994), it has been shown to induce epidermal thickening, enhance expression of CRABP II and CRBP much like retinoic acid, but shows reduced signs of erythema and irritation to tretinoin (Kang et al, 1995). Retinol also produces less trans epidermal water loss, erythema and scaling than retinoic acid, at the same time as stimulating collagen synthesis and reducing matrix metalloproteinase (MMP) production (Fisher et al, 1997; Fluhr et al, 1999).
A review of literature shows that the vehicle used for retinol delivery plays a crucial role in eliciting efficacy due to the inherent instability of retinol and its capacity to degrade to biologically inactive forms on exposure to oxygen and light. All too frequently, retinols are formulated with inactive esters such as retinyl palmitate, and the concentration of retinol is often not on the packaging, so, combined with formulation issues of stability, it is difficult for the consumer to judge which retinol contacting products will be most effective (Baumann, 2009).
Adapalene
The first third generation retinoid to gain approval is adapalene, approved for the treatment of acne. It reacts purely with RARs and not with RXRs. Much of the research in the past 10 years has focused on ingredients that bind to target certain plasma-binding proteins and therefore can be tailored to mitigate irritation traditionally associated with retinoids. Adapalene has been shown to be less irritating than tretinoin (Millikan, 2000). Adapalene is available as Differin, and is available in 0.1% lotion and 0.3% gel forms. Chandararatna showed adapalene to have excellent follicular penetration (Chandraratna, 1996), anti-imflammatory (Burke and Cunliffe, 1984) and comedolytic activity (Verschoore et al, 1993). Adaplaene has also been shown to be more stable than tretinoin, not breaking down in the presence of light or air (Vershoore et al, 1997).
Tazarotene
Tazarotene is available as a gel in either 0.05% or 0.1% formulations, and is indicated for plaque psoriasis and sometimes also prescribed for acne. It is also used in cases of photodamage. It differs from tretinoin in that tretinoin binds to both RARs and RXRs, whereas tazarotene binds only to certain RARs and not to RXRs. In modulating the expression of retinoid-responsive genes, tazarotene has the capacity to regulate cell proliferation, cell differentiation and inflammation, as well as down regulating keratinocyte expression (Nagpal et al, 1995). A comparative study of adapalene and tazarotene looked at every-other-day tazarotene versus daily adapalene due to the increased irritation seen with tztarotene, but concluded that the results were comparable in terms of efficacy (Kakita, 2000). A further double-blind, randomised, multi-centre, 24 week study (n=173) from 2004 comparing the use of tretinoin 0.05% and tazarotene 0.1% daily in the evening in photoageing showed a statistically significant benefit of tazarotene over tretinoin at 16 weeks for treatment success, photodamage, fine wrinkling, coarse wrinkling and mottled hyperpigmentation at various time points in the study, the speed of improvement was greater with tazarotene. There were no statistically relevant differences in subjects achieving at least a grade I improvement in lentigines, appearance of pore size, irregular depigmentation, elastosis, tactile roughness, telangiectasia or actinic keratosis. Cosmetic acceptability and tolerability were similar in both groups; however, tazarotene was associated with a transiently higher incidence of a burning sensation on the skin in the first week of treatment but not thereafter.
Tazarotene 0.1% cream can offer superior efficacy over tretinoin 0.05% emollient cream in the treatment of facial photodamage, particularly with respect to the speed of improvement (Lowe et al, 2004).
Adverse effects of retinoids
The retinoid reaction is a term coined to describe typical responses of pruritis, burning sensation at treatment sites, peeling and erythema (Oda et al, 1996; Kim et al, 2003). The type and dose of retinoid is known to affect the severity of adverse event (Griffiths et al, 1993); however, the efficacy in treatment of photoageing does not appear to be dose dependent: in a 99 patient, 48-week study, Griffiths concluded that the separation between clinical improvement and irritation suggest that mechanisms other than irritation dominate tretinoin-induced repair of photoageing in humans (Griffths et al, 1995). Baumann advised patients to use topical retinoids less frequently and at the lowest dose, increasing frequency of application gradually to once nightly, then increasing the dose gradually with an aim of moving to a prescription retinoid. According to Baumann, this leads to increased compliance, as well as improved efficacy of the topical retinoids. The authors also comment that use of hydroxyacids has been shown to exhibit less irritation, and this is believed to be as a result of the improved barrier function seen in long-term use of hydroxyacids (Baumann, 2009).
Orally administered retinoids have been associated with teratogenicity, and the potential absorption of retinoids in topical application during pregnancy has been debated hotly. Although there are multiple studies demonstrating safety (Jick and Terris, 1993), Baumann encourages caution and ceasing use in patients who are pregnant or breast feeding (Baumann, 2009).
Delivery systems
The use of delivery systems may enhance the efficacy of retinoids, at the same time reducing the ‘retinoid effect’. There are controlled release systems, implants, transdermal delivery systems, submicronic emulsions and vesicular carriers (Mukherjee et al, 2006). Pharmaceutical use of nanoparticles has been reported from several decades, but use of nanoparticles in topical delivery systems is relatively new. In a study published in 2002, Wissing and Muller reported a comparison of two different formulations (solid lipid nanoparticles and conventional oil and water emulsion) as carrier systems for the molecular sunscreen oxybenzone. The release rate could be decreased by up to 50% with the solid lipid nanoparticles formulation. They showed that solid lipid nanoparticles improved both distribution characteristics and stability (Wissing and Müller, 2002). Mukherjee suggested that the use of nanoparticles may optimise topical retinoid treatment. Nanoparticles are solid colloidal particles ranging in size from 1 to 1000 nm that are used as drug delivery agents and this technology can be used where a therapeutic moiety can be entrapped, adsorbed, or covalently attached and has been adapted for topical retinoid therapy (Lockman et al, 2002; Mukherjee et al, 2006).
Further work has been published looking specifically at nanoparticle delivery systems for retinoids. Jenning reported in various papers in 2000 and 2001 on SLN for retinol demonstrating improvement in stability, as well as improved localisation and controlled release of active retinol. In 2004, Patravale investigated SLN-based tretinoin gel, showing improved tolerability and stability, and, in 2005, Yamaguchi investigated a novel drug delivery system for improved all-trans retinoic acid (atRA) therapy for external treatments of photo-damaged skin. Irritation and inflammation associated with atRA therapy were substantially reduced and clinical changes in photodamage were also reported, such as thicker epidermis than classical atRA treatment and a surprising boost in production of hyaluronan among the intercellular spaces of the basal and spinous cell layers in epidermis (Yamaguchi et al, 2005).
More recently, combinations of ingredients have become available, such as the combination of retinol with neo-acetyl-glucosamine, and there are now some interesting conjugated molecules available, such as combining lactic acid with a retinoid (AHA-Ret). When applied to skin, this double-conjugated retinoid undergoes double hydrolysis to release both the collagen invigorating retinoid and the skin retexturising lactic acid. Although these two ingredients are known to be potentially irritating on their own, the natural and gradual breaking of these double bonds via hydrolysis delivers exceptional efficacy, with little-to-no irritation. AHA-Ret is formed when a single conjugate (lactyl retinoate) is combined with an AHA ester alcohol resulting in the formation of a double conjugate (ethyl lactyl retinoate, or ELR). ELR is a double conjugate retinoate ester. This new proprietary molecule differs from existing retinyl esters (e.g. retinyl palmitate) in that it undergoes a non-traditional conversion process in the skin due to double conjugation. Through double hydrolysis (breaking of molecular bonds using water), the ester bonds are gradually broken in the skin by naturally occurring enzymes or esterase.
The process of breaking these bonds gradually releases the retinoid and AHA, minimising irritation. Thus, AHA-Ret is delivered to the skin as a double conjugated, hydrolysis-based molecule that provides increased stability and reduced irritation over the parent compounds.
A study led by Us Dermatologist David McDaniel was published in 2017 in the Journal of Cosmetic Dermatology (McDaniel et al, 2017). A total of 47 patients completed the study, which clearly showed that AHA-Ret was more tolerable than either 1% retinol or 0.0.25% tretinoin.
AHA-Ret significantly reduced average severity of fine lines/wrinkles, dyschromia, skin tone degradation, erythema and pore size at 12 weeks.
AHA-Ret outperformed 1.0% retinol cream significantly inducing less erythema at 8 and 12 weeks, and significantly reducing dyschromia and skin tone degradation earlier than retinol.
AHA-Ret was non-inferior to 0.025% prescription tretinoin at 4 and 8 weeks in each category, and non-inferior for fine lines/wrinkles, dyschromia, skin tone degradation, and erythema at 12 weeks.
Treatment with a double-conjugate retinoid cream achieved early, progressive improvements in the visible signs of ageing, representing a new advancement in skin rejuvenation.
Conclusion
Topical Vitamin A lies in the realm of ‘tried and trusted’ ingredients. The new combination formulations, such as Retinol and neoglucosamine, and combination molecules, such as a retinoid with lactic acid (AHA-Ret), pave the way for progressive improvement in patient outcomes.


