
Periocular rejuvenation represents one of the leading concerns for aesthetic patients. A European market study (2020) of 27283 participants found that 75% of consumers experienced undereye issues ‘always or often’ (Eyelight, 2020). Additionally, 66% of consumers are bothered by undereye circles (Eyelight, 2020). The consumer study also found that, regardless of gender, 70% of participants felt they looked tired or older than they are due to undereye circles (Eyelight, 2020). This is no surprise; as human beings, the eyes are the first facial feature looked at when a human face is seen (Chatterjee et al, 2009). Furthermore, looking into someone's eyes stimulates areas in the brain for both perception of beauty and facial recognition (Chatterjee et al, 2009). Possessing the ability to safely offer periocular rejuvenation within a clinical practice is of paramount importance. Within a recent consumer study, 69% of participants have had or considered a treatment for dark circles and/or eye bags (International Society of Aesthetic Plastic Surgery, 2020). Infraorbital hollowing can give patients a fatigued appearance despite having sufficient rest. In the author's experience, treatment for the infraorbital hollow has high satisfaction ratings and can give patients an immediate aesthetic improvement. Treating this area requires detailed knowledge of anatomy in combination with an injection technique intimately related to the rheology of the dermal filler used.
The term ‘tear trough deformity’ was first coined by Flowers in 1993 (Berguiga and Galatoire, 2017). The tear trough is defined as the depression of the medial lower eyelid just lateral to the anterior lacrimal crest and limited in its inferior aspect by the inferior orbital rim. It is limited in its lateral extent by the mid pupillary line.
There are a number of age-related changes that occur in the periorbital area. Some changes warrant tear trough augmentation and others are a contraindication for treatment. Some of these changes are present genetically in youth. Skin changes occur in the periorbital area, notably with a loss of volume, reduction in the amount of collagen and distortion of elastin fibres resulting in a decrease in elasticity (Stutman and Codner, 2012). Thinning and descent of the facial fat pads and prolapse of the suborbicularis oculi fat (SOOF) both contribute to the formation of a tear trough deformity. Widening of the orbits due to recession of the inferior rim contributes to the prolapse of fat pads and, subsequently, reveals a tear trough deformity. (Stutman and Codner, 2012).
Rejuvenating the tear trough with hyaluronic acid filler
Before deciding to treat the tear trough area, it is important to have a system for patient selection. The author's preferred grading system for the tear trough area is Belhaouari et al's (2014) classification. This is because it not only provides an anatomical analysis but also gives practitioners a therapeutic guide:
- Stage 1A: insufficient volume without ptosis
- Stage 1B: insufficient volume with ptosis
- Stage 2A: normal volume without ptosis
- Stage 2B: normal volume with ptosis
- Stage 3A: increased volume without ptosis
- Stage 3B: increased volume with ptosis.
A direct approach to treating the tear trough is indicated in patients without ptosis of the infraorbital fat (1A, 2A and 3A). An indirect approach is required for patients with ptosis (1B, 2B and 3B), restoring the temple and malar region to provide mechanical support to the tear trough (de Maio, 2020).
In the author's opinion, the assessment for patient suitability of direct tear trough injection is the most important skill when augmenting this area. A full medical history must be taken, paying attention to any previous injection of products in the area. Previous permanent products, such as silicone injections, are a contraindication.
The patient must be assessed in an upright position. An upwards gaze reveals the tear trough deformity and potential ptosis. The author always assesses the lower eyelid hyperlaxity using the snap-back test. If positive, this is a relative contraindication. It is also important to establish the presence of periocular fluid retention, which is an absolute contraindication for treatment. Manual pressure on the globe will uncover a weak septum, which predisposes to fat pad prolapse in the future and will help plan future treatments.
Complications in the tear trough area are largely made up of injection-related reactions, such as erythema, oedema, pain, bruising and itching. In a retrospective study of 151 patients with dermal filler placed preperiosteally, deep to the orbicularis, anterior to the inferior orbital rim, the most common side effects observed after injection included bruising (11%), swelling (12%) and redness (inflammation 12%). Very few incidents of Tyndell effect (2.6%) were observed (Belhaouari and Galatoire, 2017). Incidence of irregular correction has been found to be variable in literature but found to be as high as 33% with some hyaluronic acid fillers (Morley and Malhotra, 2011). This highlights the need for a safe injection protocol. Excessive oedema was found in 0.9% of cases and was present when the injection was performed in the superficial plane with too much product placed and or high extrusion force (Belhaouari and Galatoire, 2017).
Filler rheology
The author's product of choice in the tear trough area is Restylane Eyelight. The rheology of the product is designed specifically for the tear trough. This is because of the patented non-animal stabilised hyaluronic acid (NASHA) technology. This firmer gel allows precision, projection and targeted product integration. These are ideal qualities for a tear trough filler. It gives the injector the assurance that there will be limited migration of product into the surrounding tissues and stays in the area injected, targeting and treating the cause of the tear trough deformity upon examination of the patient. Restylane Eyelight is indicated and licensed for use in the periorbital area, administered as deep injections to the bone or into the subcutaneous fatty tissue (Nikolis et al, 2021). NASHA's patented hyaluronic acid stabilisation technique preserves natural cross-links and entangled hyaluronic acid networks (Nikolis et al, 2021).
Injection protocol
The entry point for augmentation of the tear trough can be found easily as the intersection between two lines; one line represents the projection of the nasojugal fold, and the second is the perpendicular projection of the lateral canthus of the eye. Identification of the infraorbital foramen is advised to prevent complications. A 25 gauge cannula is carefully inserted deeply through the superficial musculoaponeurotic system (SMAS). The tip of the cannula should gently touch the periosteum. Note that slow movements are imperative in this area to prevent trauma to the lymphatic system and neurovascular structures. The cannula is gently moved towards the medial canthus in the direction of the tear trough. At this stage, the author finds it useful to get the patient to adopt an upward gaze to allow smooth passage of the cannula. As the mid pupillary line is approached, there is normally some resistance; this is due to the presence of the orbitomalar ligament. At this point, the cannula should not be advanced forward. Most practitioners would agree that filler should be placed supraperiosteally (Kane, 2005); however, given the low hygroscopic behaviour of Restylane Eyelight, the author believes it is safe to place filler in the deeper layers of the SOOF (Chang et al, 2012).
The filler is gently placed in an intermittent retrograde microbolus fashion along the length of the tear trough at the level of the orbital rim. The product is then gently massaged for homogenisation. Given the rheology of the product (higher G-prime), a maximum of 0.25ml for each tear trough is recommended.
Post-procedure, the author recommends that patients avoid strenuous physical exercise, as well as compressing the area. A follow-up visit is normally scheduled for 2 weeks post procedure. In the author's clinical practice, the addition of more dermal filler is restricted to 3 months post-injection.
Restylane Eyelight
NASHA technology offers an established safety profile in the tear trough across numerous studies (Nikolis et al, 2021). Restylane Eyelight demonstrates a favourable clinical safety profile in the periorbital area (Nikolis et al, 2021). Some 42 patients reported adverse events at week 2 and week 4 following two treatments of infraorbital hollowing with Restylane Eyelight (Figure 1).
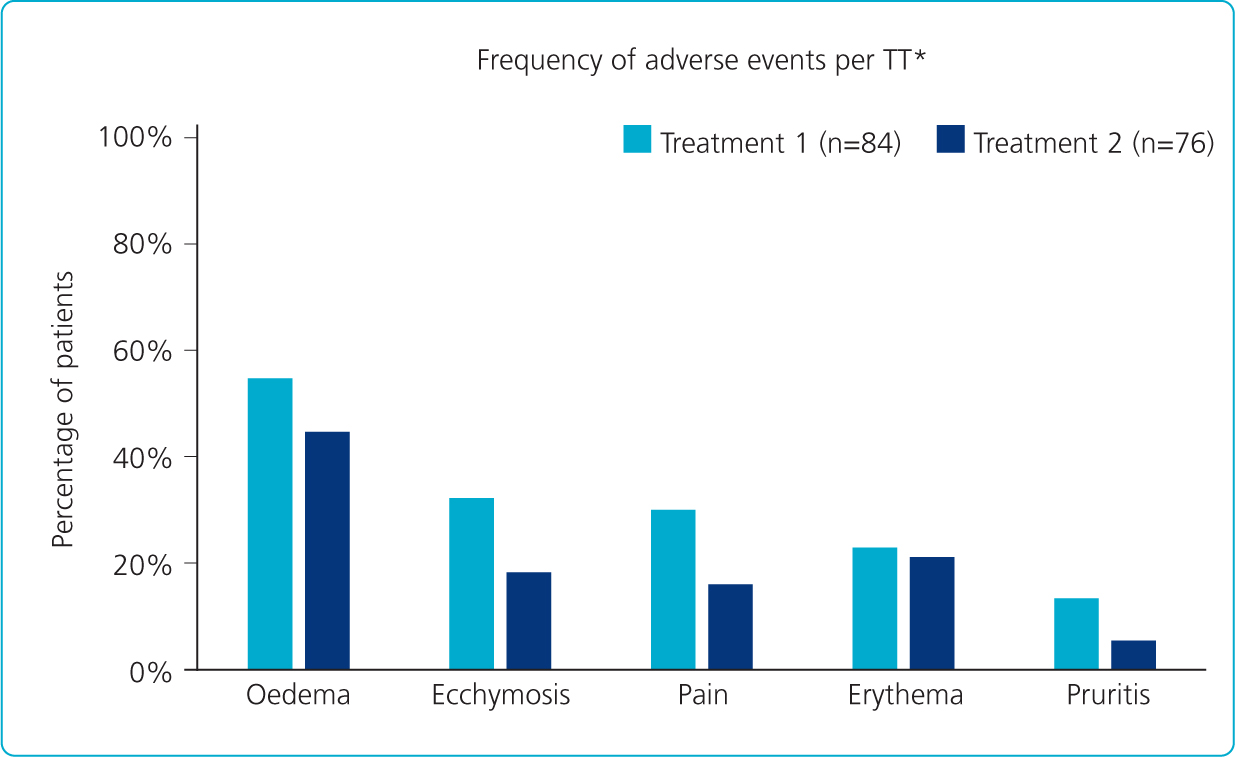
The adverse events reported were mild to moderate in severity, transient and expected following HA filler injection (i.e. related to the injection technique rather than Restylane Eyelight) (Nikolis et al, 2021). It is also worth noting that a large proportion of the patients (21 for treatment one and 18 in treatment two) had their tear trough filler administered with a needle, which would provide an explanation for the higher incidences of oedema and bruising.
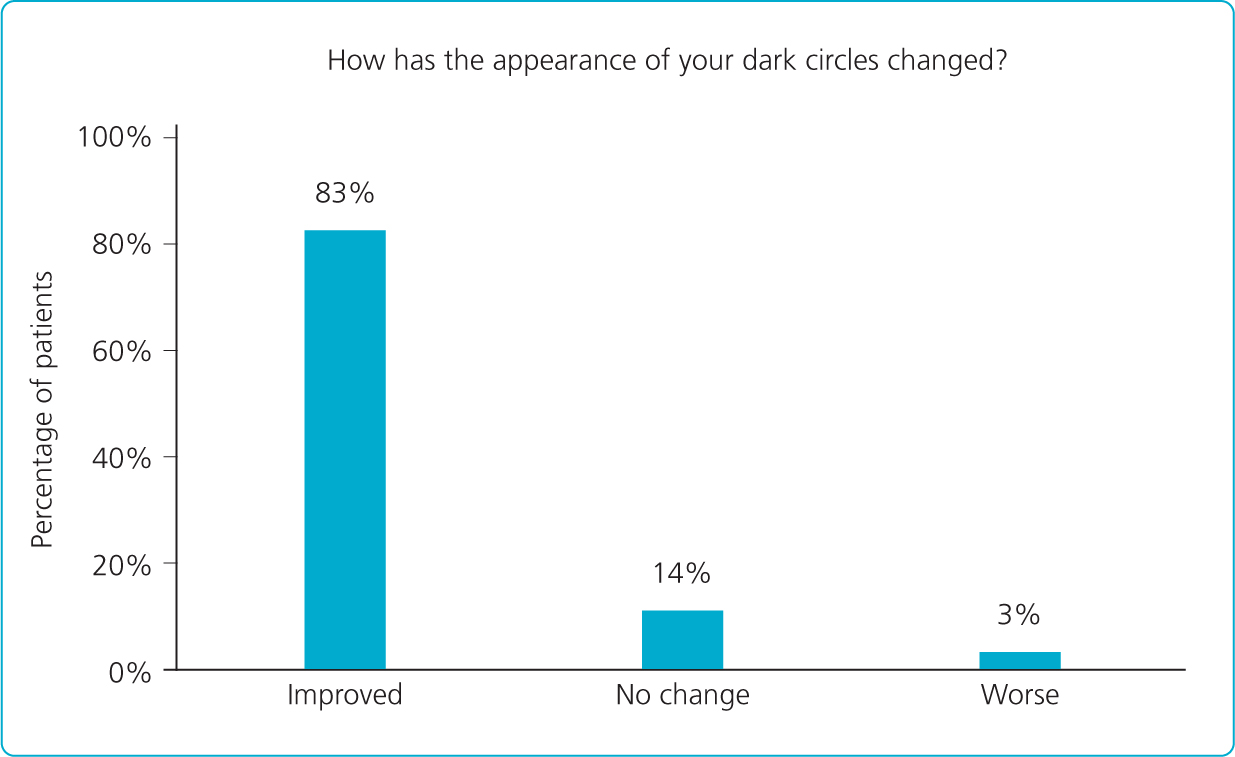
More importantly, 83% of patients felt that the treatment had improved the appearance of their dark circles (Figure 2). Patients observed significant improvements in their appearance more than 6 months after treatment with Restylane Eyelight, with 88.6% of treated patients reported as looking less tired 6.5 months after their treatment (Nikolis et al, 2021).
Tear trough rejuvenation case studies using Restylane Eyelight
Patient one
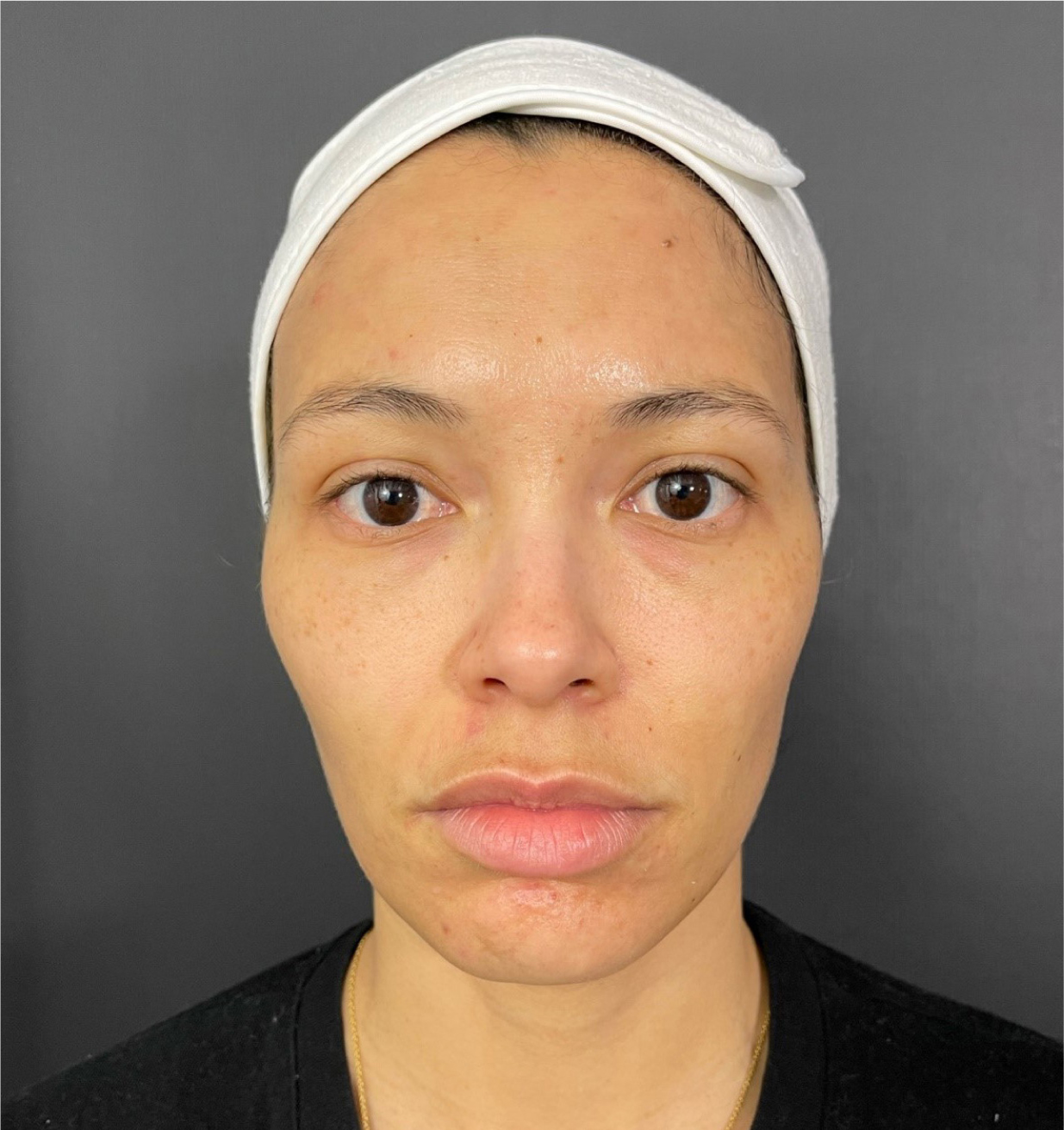
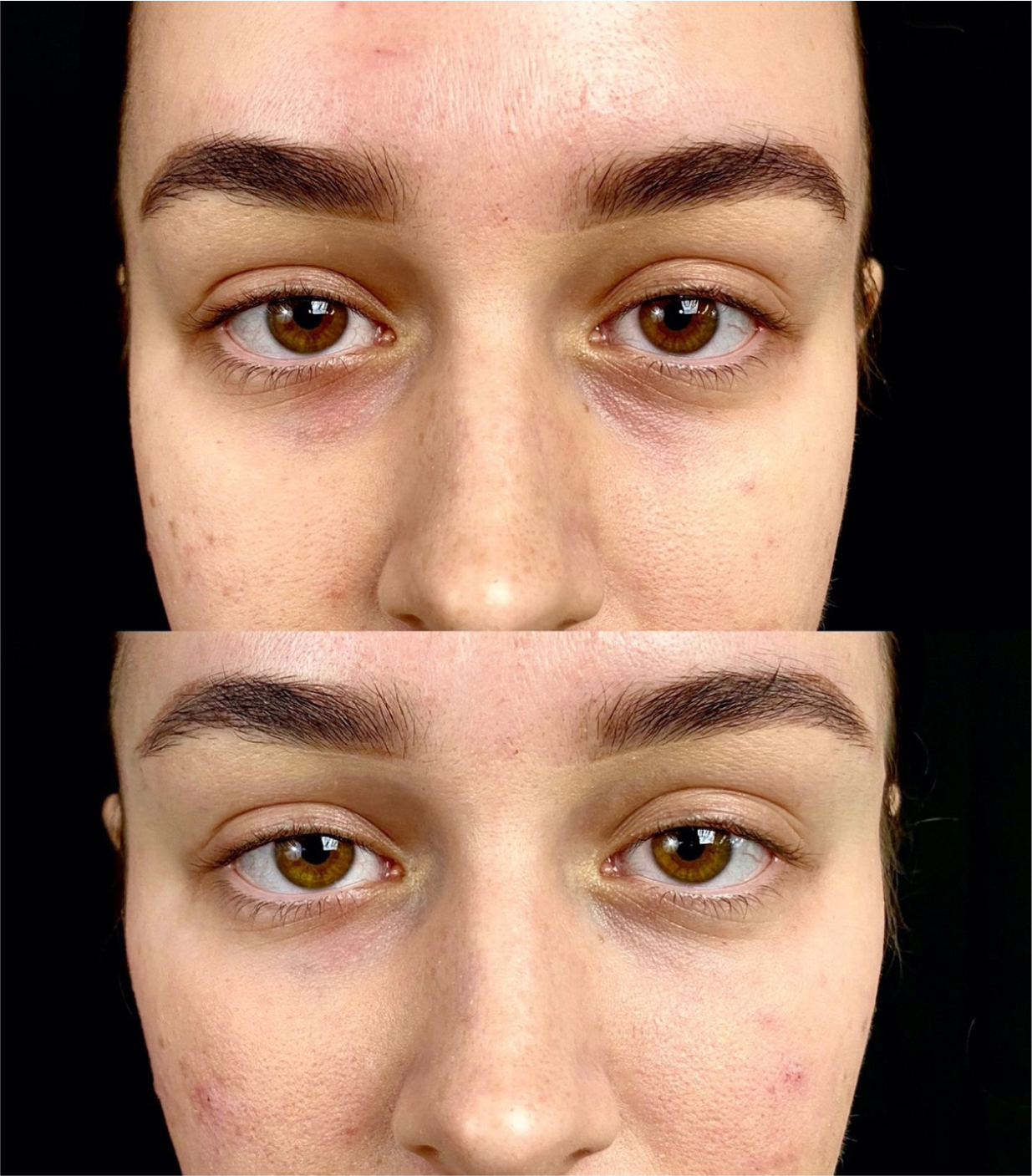
This was a 31-year-old female patient with no medical history and no previous injectable treatments. Upon examination, the right side was classified as 1A (insufficient volume without ptosis) and the left side 1B (insufficient volume with ptosis) without impaired lymphatic drainage (Figure 3). She complained of looking tired and noted that her left eye was her ‘more tired’ side. The author performed a direct tear trough treatment with a 25G cannula, injecting 0.1ml of Restylane Eyelight supraperiosteally on the right side in an intermittent retrograde fashion. On the left side, an indirect treatment was performed, adding Restylane Volyme to the anterior and lateral malar areas, as well as Restylane Eyelight in the anatomical tear trough (0.25ml).
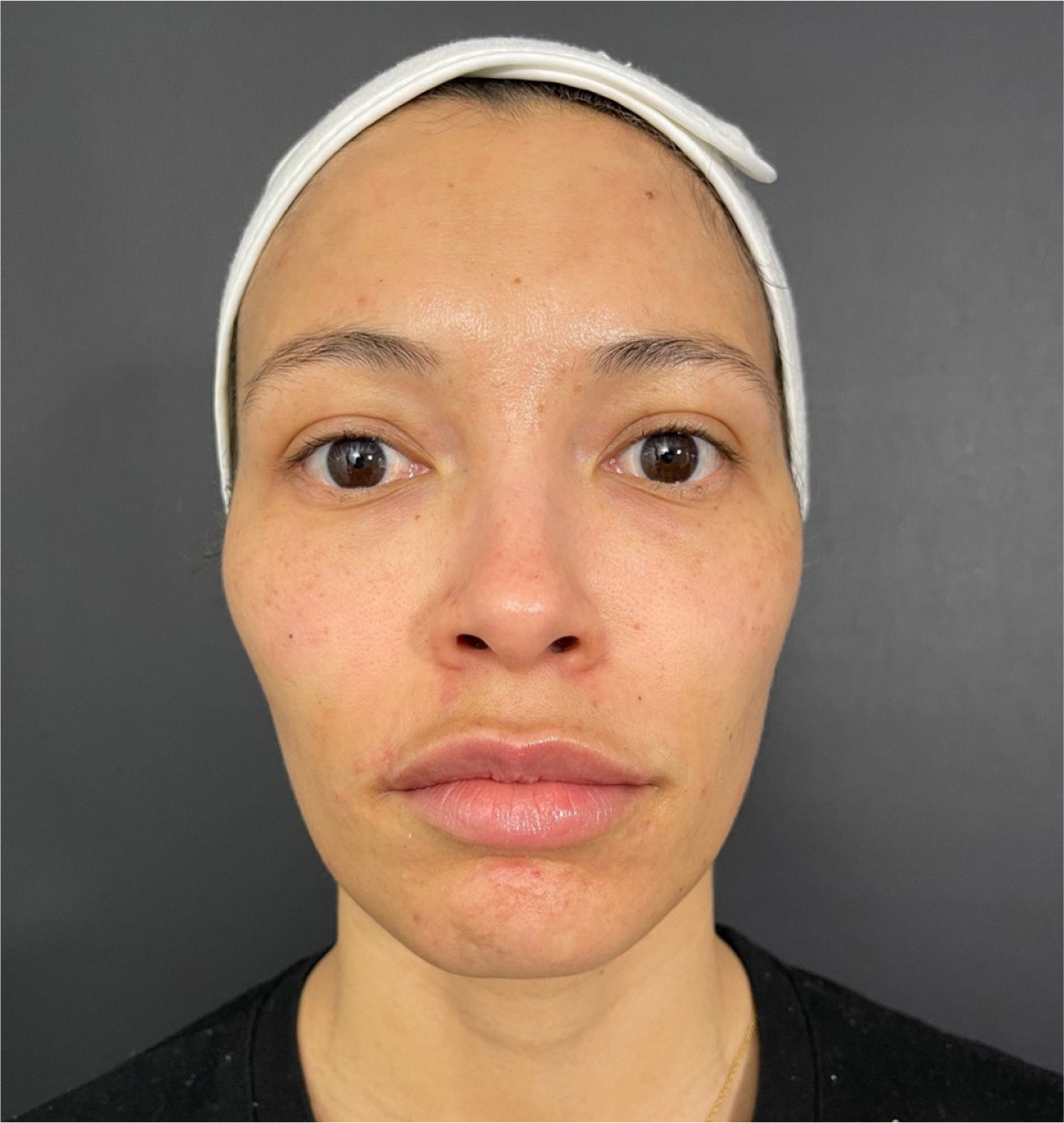
Post procedure, an immediate correction of the tear trough can be observed, with minimal oedema and ecchymosis (Figure 4).
Patient two
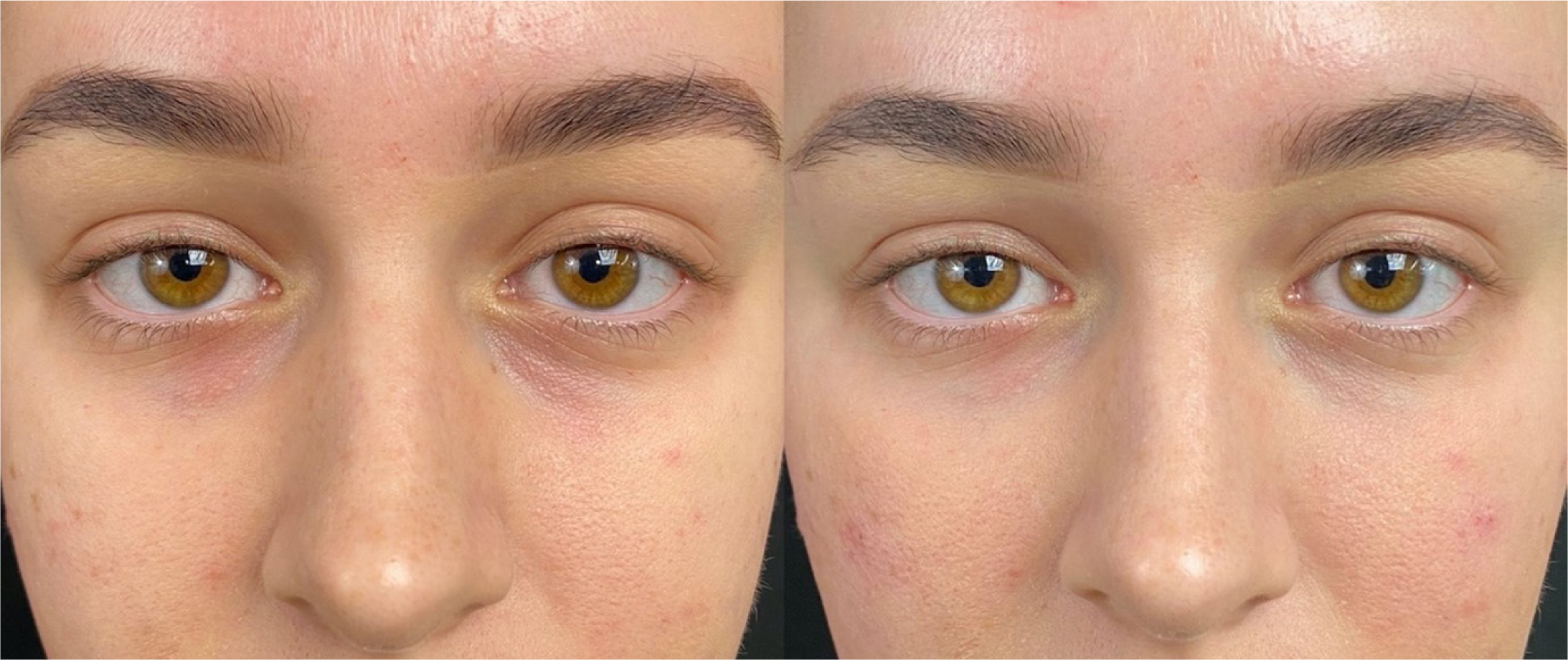
A 23-year-old female patient presented with an anatomical tear trough deformity and complained of dark eyes and hollowness. There was no previous medical history. Tear trough augmentation was performed bilaterally with a 25G cannula using Restylane Eyelight. Dermal filler was placed deep on the bone up to the orbitomalar ligment in the medial extremity and at the end of the tear trough laterally. This resulted in an immediate improvement in the tear trough deformity (Figures 5 and 6).
Patient three
This 40-year-old patient presented with a type 1A tear trough deformity, complaining of darkness and a lack of freshness under the eye (Figure 7). She had no previous medical history. The patient had undergone previous dermal filler in the perioral region 2 years previously. After explaining to the patient that the darkness was a combination of pigmentation, as well as shadowing created by the subtle tear trough deformity, a tear trough augmentation was performed using a single bolus of Restylane Eyelight 0.05ml supraperiosteally bilaterally using a 25G cannula. The result was an immediate aesthetic improvement of the area (Figure 8).


Conclusion
In summary, the use of dermal filler to correct the tear trough deformity is a safe non-invasive procedure that is increasingly in demand. Given the results of the aforementioned consumer study by Eyelight (2020), it is imperative that aesthetic practitioners incorporate Restylane Eyelight and tear trough rejuvenation into the patient journey. Seeking further training in injection techniques and revisiting the anatomy of the area is of paramount importance. In this article, the author has constructed a small guide to start treating the tear trough. The use of Restylane Eyelight reduces the risk of over-injecting the area, given the fact that it is supplied in a 0.5cc syringe. It is important to understand that, although this is a lower quantity in comparison to other licensed tear trough products on the market, the unique rheology of the hyaluronic acid gel sets the product apart. Given the higher G-prime, a smaller quantity can be injected for maximal effect. Targeted product integration helps reduce the theoretical risk of migration of dermal filler both months and years post injection. An assessment of the skin quality and amount of underlying subcutaneous fat is essential when using Restylane Eyelight, as an injection in the more superficial compartments could lead to injury of lymphatics, as well as contour irregularities. Adequate assessment also helps to avoid potential complications. Beljaouari et al (2014) provided a framework to categorise tear troughs with possible treatment options. If just beginning to treat the tear trough area in patients, the author would recommend avoiding patients with ptosis and/or excessive volume in the tear trough. ‘Less is more’ is always the best strategy in this delicate area. Looking back at a decade of treating the tear trough, the author can conclude that it is essential to undercorrect the tear trough.
In conclusion, the use of Restylane Eyelight to correct tear trough deformity ensures enhanced patient satisfaction beyond 6 months post injection. Crucially, using Restylane Eyelight in this area is related to a low incidence of transient injection-related side effects (Nikolis et al, 2021).



